News

News

News

Medical devices regulation

News

Medical devices regulation

Medical devices regulation

Medical devices regulation

Medical devices regulation
News

News

News

Medical devices regulation
News

Medical devices regulation

Medical devices regulation
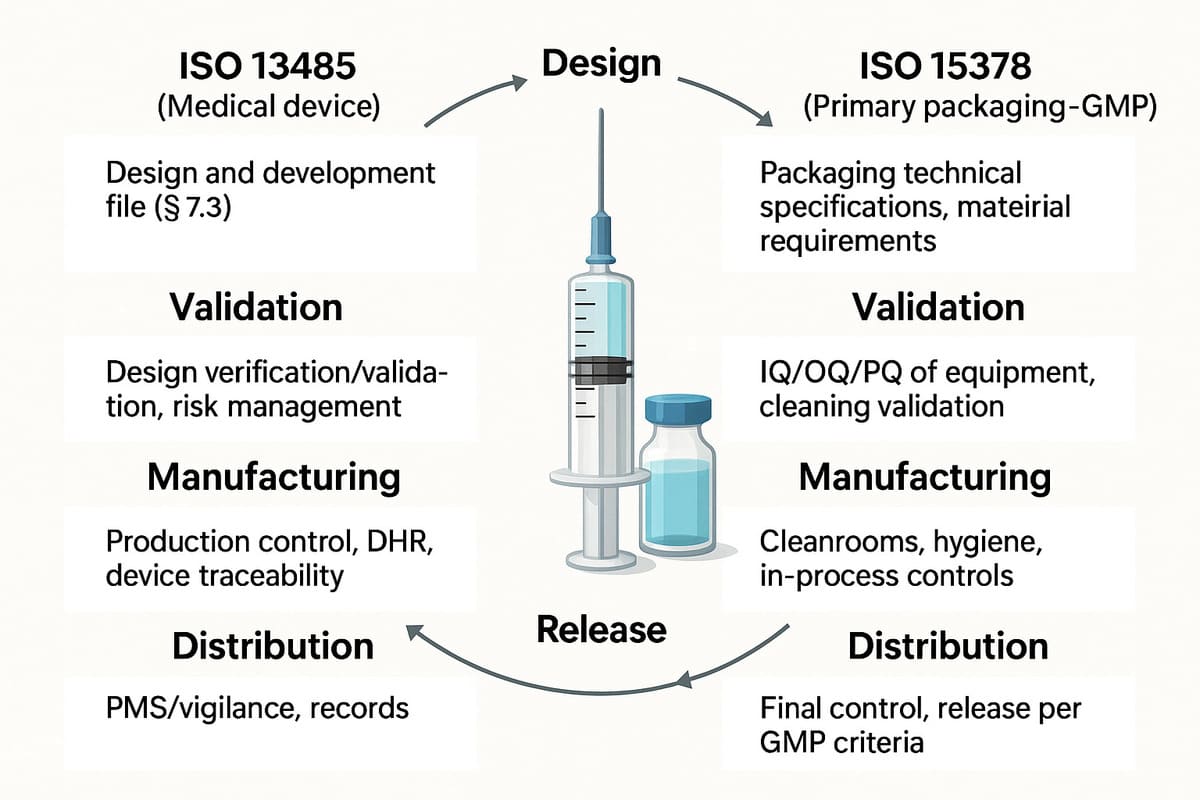
Medical devices regulation

News

News

News

Medical devices regulation