News about medical devices
News
Fitness for use and risk management: how a manufacturer of motorized dental instruments secured its CE/FDA file thanks to an integrated approach

News
MDR harmonized standards published in January 2026: what manufacturers really need to look at

News
The European Commission wants to simplify MDR and IVDR: what do manufacturers really need to understand?
News
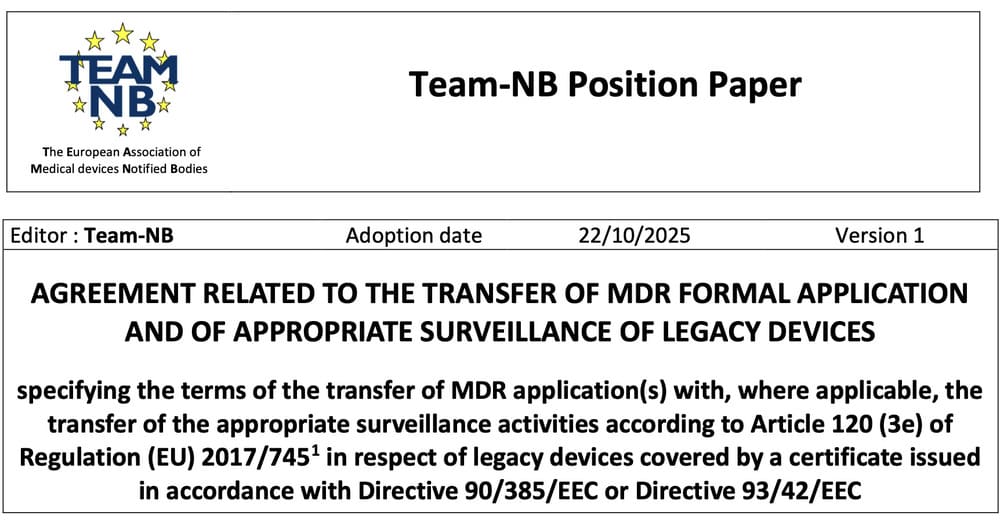
MDR: what does the transfer agreement published by Team-NB really change?

News
Pilot coordinated assessment for CI/PS: a new step towards European coordinated assessment of clinical studies

News
MedTech start-ups: how to get a CE marking project off to a good start?
News
ISO 13485 for start-ups: 3 realistic approaches

News
FDA vs MDR: 5 differences that matter to DM manufacturers

News
IVDR and IVD Software: How to Properly Qualify and Classify Your Software

News
CECP Procedure: What Should Medical Device Manufacturers Watch Out For?
News
MDR CE marking: pitfalls to avoid as a start-up

News
ISO 14971 and start-ups: how to get started on risk analysis without getting lost

News
MDR technical file submission: what manufacturers really need to take care of (Team-NB, April 2025)

News
MD or not MD? What the ANSM guide to borderline cases really says (April 2025)

News
Medicinal products used with a medical device: what does the EMA guideline on quality documentation have to say?
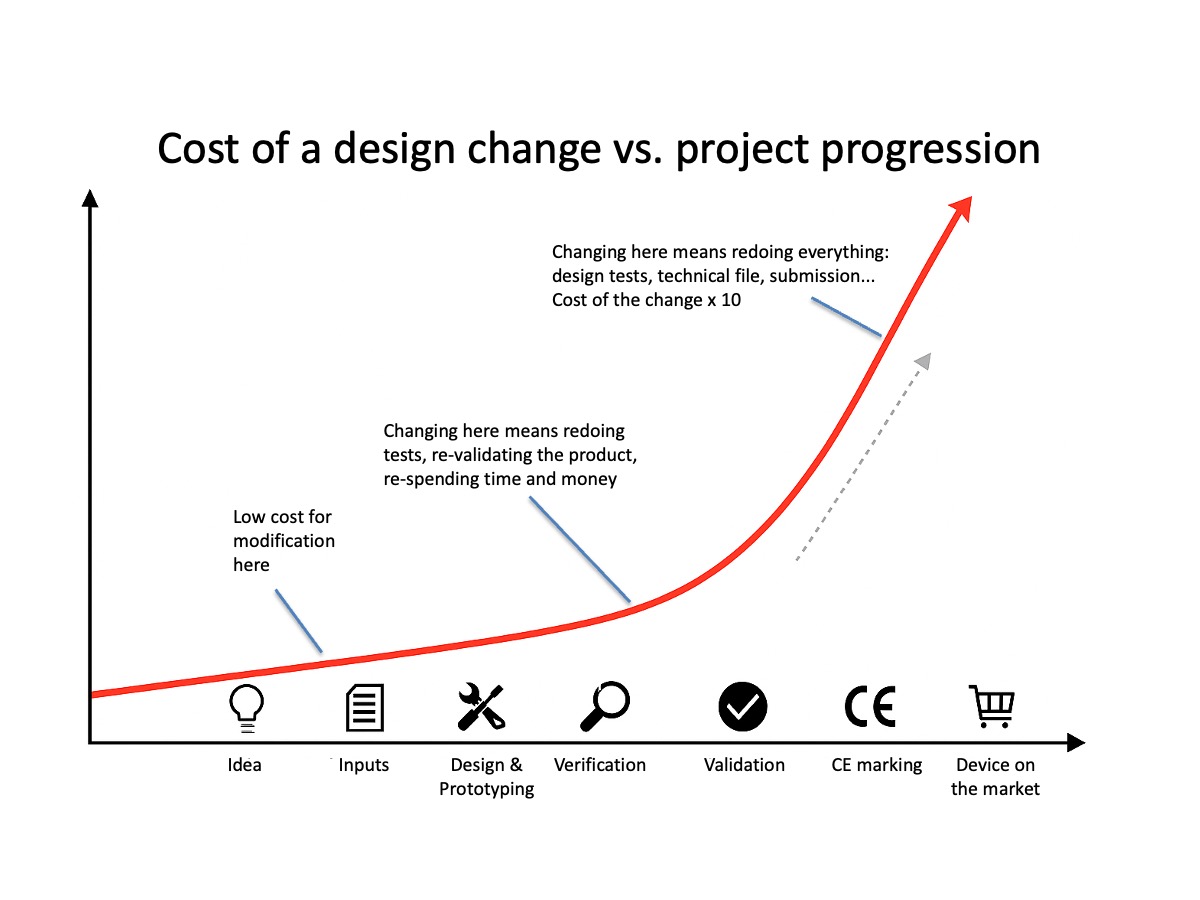
News
What is good regulatory support for a start-up?

News
MedTech start-ups: where to begin preparing for CE marking?

News
Practical Guide: Submitting Technical Documentation under MDR (EU) 2017/745

News
Urgent Revision of MDR and IVDR in 2024: What Manufacturers Need to Know

News
Sun Tzu and Medical Devices




















