News about medical devices
MD, AI and Cybersecurity
AI Act and medical devices: what manufacturers need to anticipate (Team-NB, 2025)

MD, AI and Cybersecurity
Characterizing the risks of medical software: what should we learn from IMDRF guide N81 (2025)?

MD, AI and Cybersecurity
Characterizing the risks of medical software: what should we learn from IMDRF guide N88 (2025)?

MD, AI and Cybersecurity
Simplify Regulatory Compliance for AI-Powered Medical Devices with the Team NB and IG-NB Questionnaire

MD, AI and Cybersecurity
The AI risk repository

MD, AI and Cybersecurity
Successful and timely uptake of artificial intelligence in science in the EU

MD, AI and Cybersecurity
Statement of Applicability according to section 6.1.3 of ISO/IEC 42001:2023

MD, AI and Cybersecurity
How far can data support the transformation of health through AI?

MD, AI and Cybersecurity
NIS 2 Directive
MD, AI and Cybersecurity
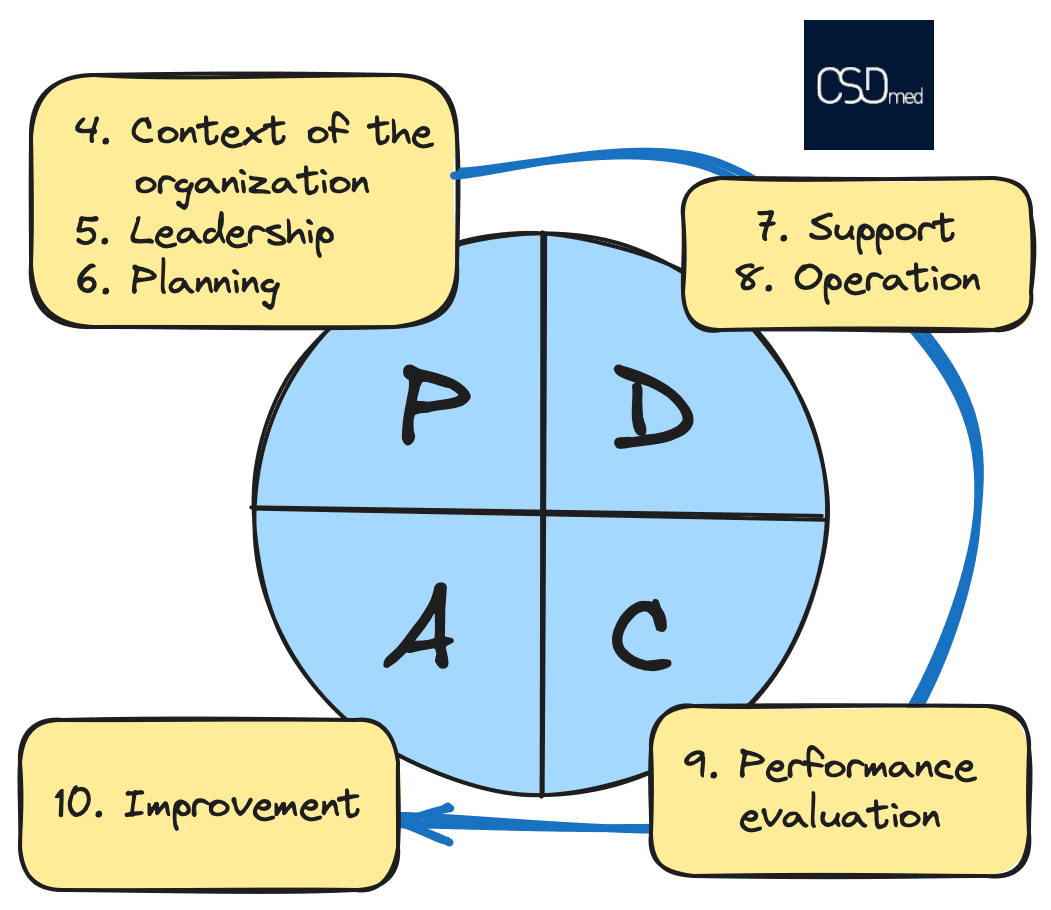
HLS structure within ISO-IEC 42001

MD, AI and Cybersecurity
ISO 42001:2023 Artificial intelligence - Management system











