Combination products: bridges between ISO 13485 and ISO 15378 (and what the authorities expect)
Medical devices regulation
1. When two worlds meet
Combination products are at the crossroads of two demanding regulatory worlds: medical devices and drugs.
Whether it's an auto-injector, a pre-filled syringe or a transdermal patch, these products raise a simple but strategic question: which quality standard should be applied?
ISO 13485? ISO 15378 or both? This article will help you understand the bridges between these two standards, and above all, what the health authorities and your customers really expect from them.
2. ISO 13485 and ISO 15378: two standards derived from ISO 9001, but for different needs
What they have in common :
Both are based on the logic of ISO 9001: process approach, PDCA, controlled documentation.
Both apply to regulated products.
Both require structured risk and change management.
What sets them apart :
|
ISO 13485 |
ISO 15378 |
|
QMS standard specific to medical devices |
QMS standard applied to primary packaging for medicinal products |
|
Integrates the requirements of MDR 2017/745 and authorities such as the FDA (QMSR) |
Integrates GMP (Good Manufacturing Practice) requirements expected in the pharma world |
|
Focused on the finished product (DM) |
Focuses on packaging components in direct contact with the drug |
To remember: ISO 13485 is aimed at legal manufacturers of medical devices; ISO 15378 is aimed at pharmaceutical suppliers of critical packaging. But the boundaries become blurred in the case of combination products.
3. Which cases require one, the other... or both?
Here are a few typical cases to get you started:
|
You are.. |
ISO 13485 |
ISO 15378 |
Certification required? |
Remarks |
|
Holder of a combined product (AMM + CE marking) |
✅ |
✅ |
Not necessarily both, but justification required |
Double conformity expected: DM + drug |
|
Manufacturer of administration device (auto-injector, pump, patch...) |
✅ |
⚠️ if drug contact |
Yes (ISO 13485) |
ISO 13485 required if device is designed or modified |
|
Manufacturer of primary packaging (syringe, vial, cap, capsule...) |
❌ |
✅ |
Yes (ISO 15378 or equivalent GMP) |
ISO 15378 is the expected reference standard |
|
Full-service subcontractor (CDMO, OEM) |
✅ |
✅ |
Recommended, depending on role in project |
Often assessed by customer audit rather than dual certification |
|
Combination productdistributor |
❌ |
❌ |
No, but responsibilities to be verified |
Must ensure that manufacturers and CMOs are compliant |
Important: standards are not automatically mandatory, but the underlying requirements (traceability, validation, hygiene, risks...) are. Effective application must be demonstrated.
4. What the authorities expect (EU, FDA, pharma customers)
4.1. Europe (EMA + MDR)
MDR 2017/745 does not explicitly cover combination products, but requires a joint assessment (notified body + medicines competent authority) for devices incorporating a medicinal substance.
The DM manufacturer must demonstrate ISO 13485 compliance, and the drug component must comply with GMP.
4.2. on the FDA side (21 CFR Part 4)
The FDA requires simultaneous compliance with :
21 CFR Part 820 (soon to be aligned with ISO 13485) for DM
21 CFR Part 210/211 for drugs
Combination products are therefore doubly concerned. The FDA proposes a guide for allocating quality responsibilities.
4.3. Pharmaceutical customers
They generally require suppliers of packaging or critical components to be ISO 15378 certified, or to demonstrate equivalent GMP practices.
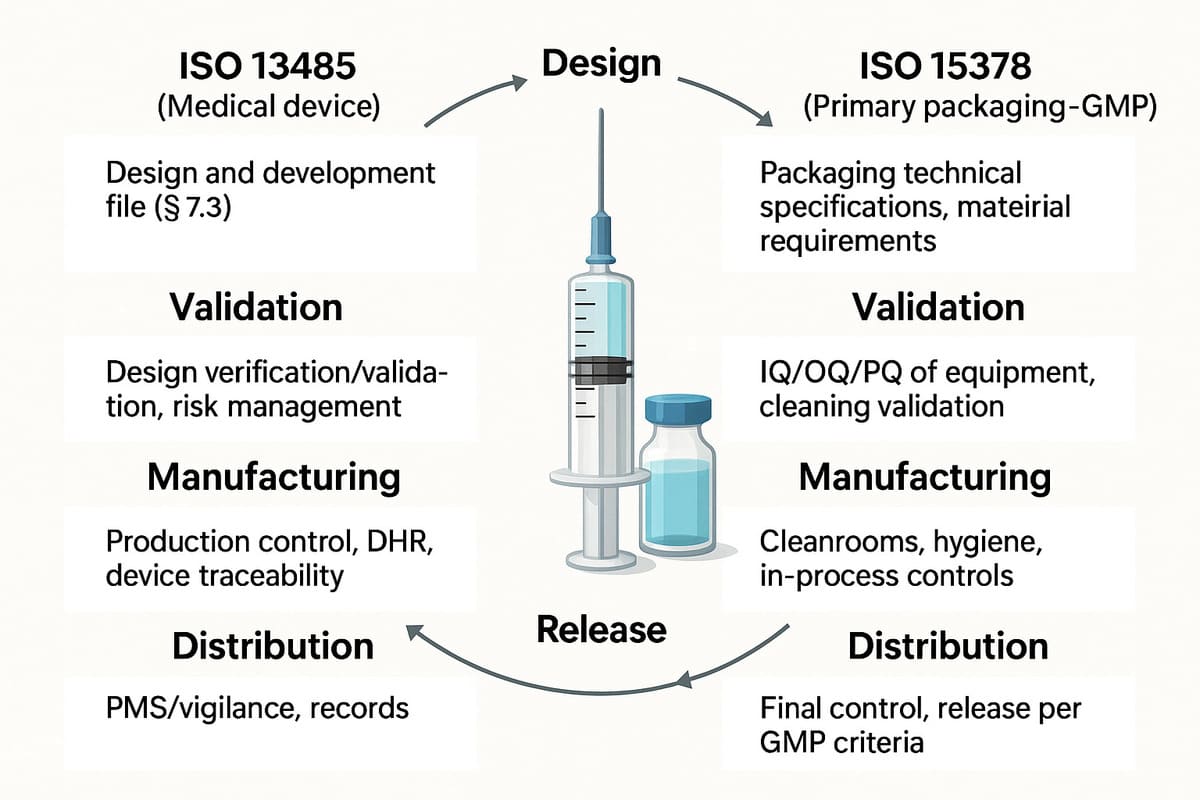
4.4. One life cycle, two quality logics to coordinate: ISO 13485 vs ISO 15378, step by step
Although ISO 13485 and ISO 15378 are based on common principles (QMS, risks, validation, etc.), they apply differently at different stages of the combined product life cycle.
Here's a cross-referenced overview of typical requirements at each stage:
|
Stage |
ISO 13485 (Medical device) |
ISO 15378 (Primary packaging - GMP) |
|
Design |
Design file (§ 7.3) |
Packaging technical specifications, material requirements |
|
Validation |
Design verification/validation, risk management |
IQ/OQ/PQ qualification of equipment, cleaning validation |
|
Manufacturing |
Production control, DHR, DM traceability |
Clean areas, hygiene, in-process controls |
|
Release |
Batch review, design conformity |
Final inspection, release according to GMP criteria |
|
Distribution |
PMS / vigilance, records |
Review of batch records, material traceability |
This dual quality management system requires coordination of documentation, operations and regulations. This is where the coherence of the overall QMS becomes a strategic issue.
5. Case studies
Case 1: Pre-filled autoinjector (biotech start-up)
The customer is developing an injectable drug, packaged in a pen injector-type device.
He designs the device himself, with a subcontracting partner.
What we apply :
ISO 13485 for the device part + CE marking.
Application of GMP requirements (by the partner manufacturing the primary packaging) → ISO 15378 or equivalent required.
Essential coordination in the technical file to justify cross responsibilities.
Case 2: Manufacturer of vials and caps for nasal devices
Supplier of molded plastic components, direct contact with the drug.
The end customer is a pharmaceutical laboratory which assembles these components with a DM.
What we apply:
ISO 15378 mandatory (material traceability, clean environment, cleaning validation).
No need for ISO 13485 unless components are claimed as DM or integral part.
These two cases illustrate how the application of ISO 13485 and ISO 15378 depends on the exact role in the value chain.
6. Mini FAQ
Do I need both certifications (13485 + 15378)?
Not necessarily. But you do need to be able to demonstrate that all requirements have been effectively implemented (including GMP).
Is ISO 9001 sufficient?
No. Neither for the device (ISO 13485 required), nor for the primary packaging (ISO 15378 or GMP).
What if I'm just distributing the product?
You won't necessarily be certified, but you must make sure that your suppliers are.
Can a company have a single QMS covering both standards?
Yes, in fact we recommend it. In this case, you need to clearly map the specific requirements in your quality documentation.
7. A dual quality culture is essential
Combined products require you to break out of your regulatory silos. It's not enough to have a "DM" or "Pharma" QMS: you need to know how to combine requirements and speak both languages.
At CSDmed, we help manufacturers to build a coherent quality strategy for combination products: from the choice of reference systems to the organization of documentation and the management of subcontractors. Let's talk about it.
8. Related resources