
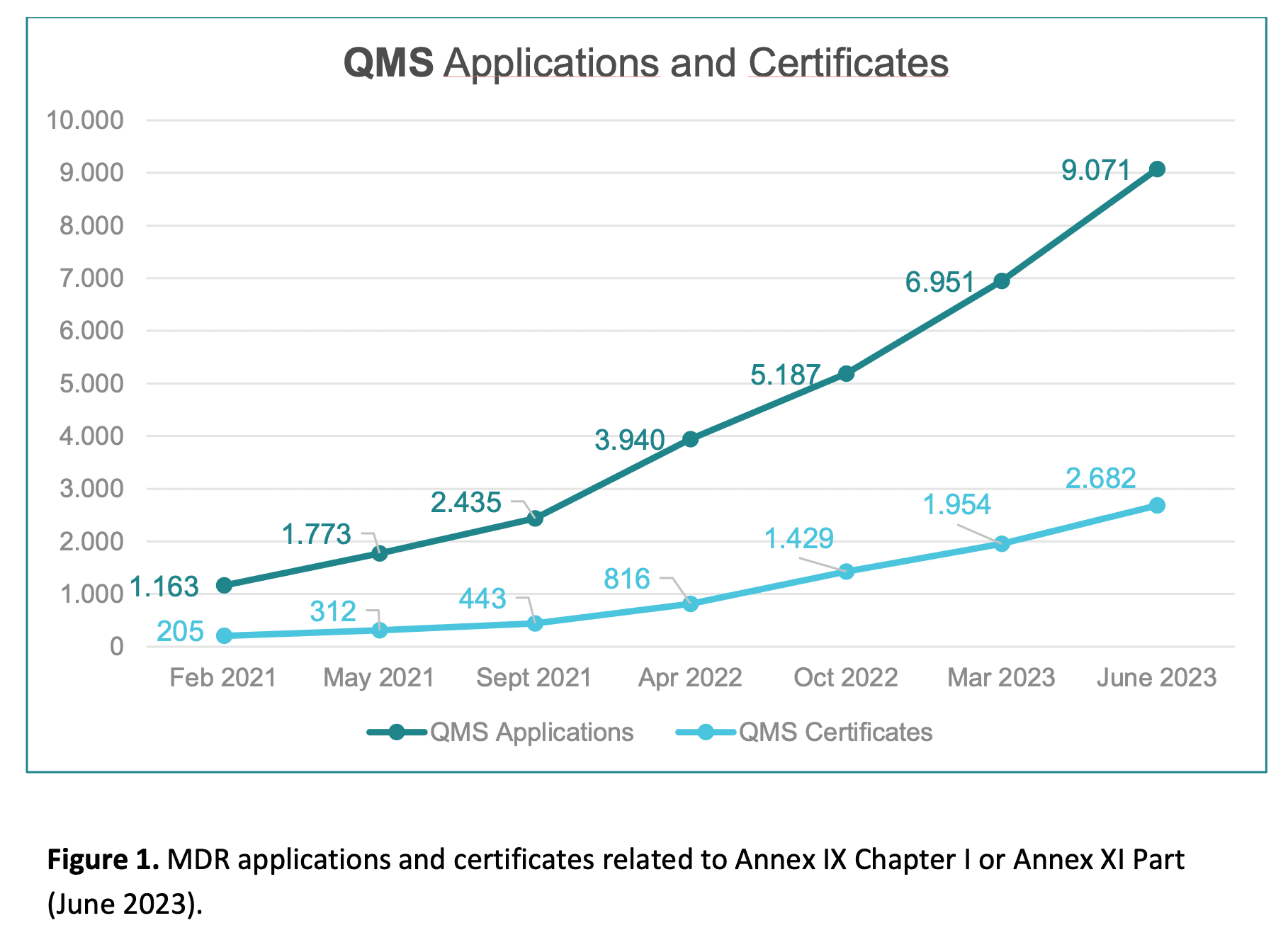
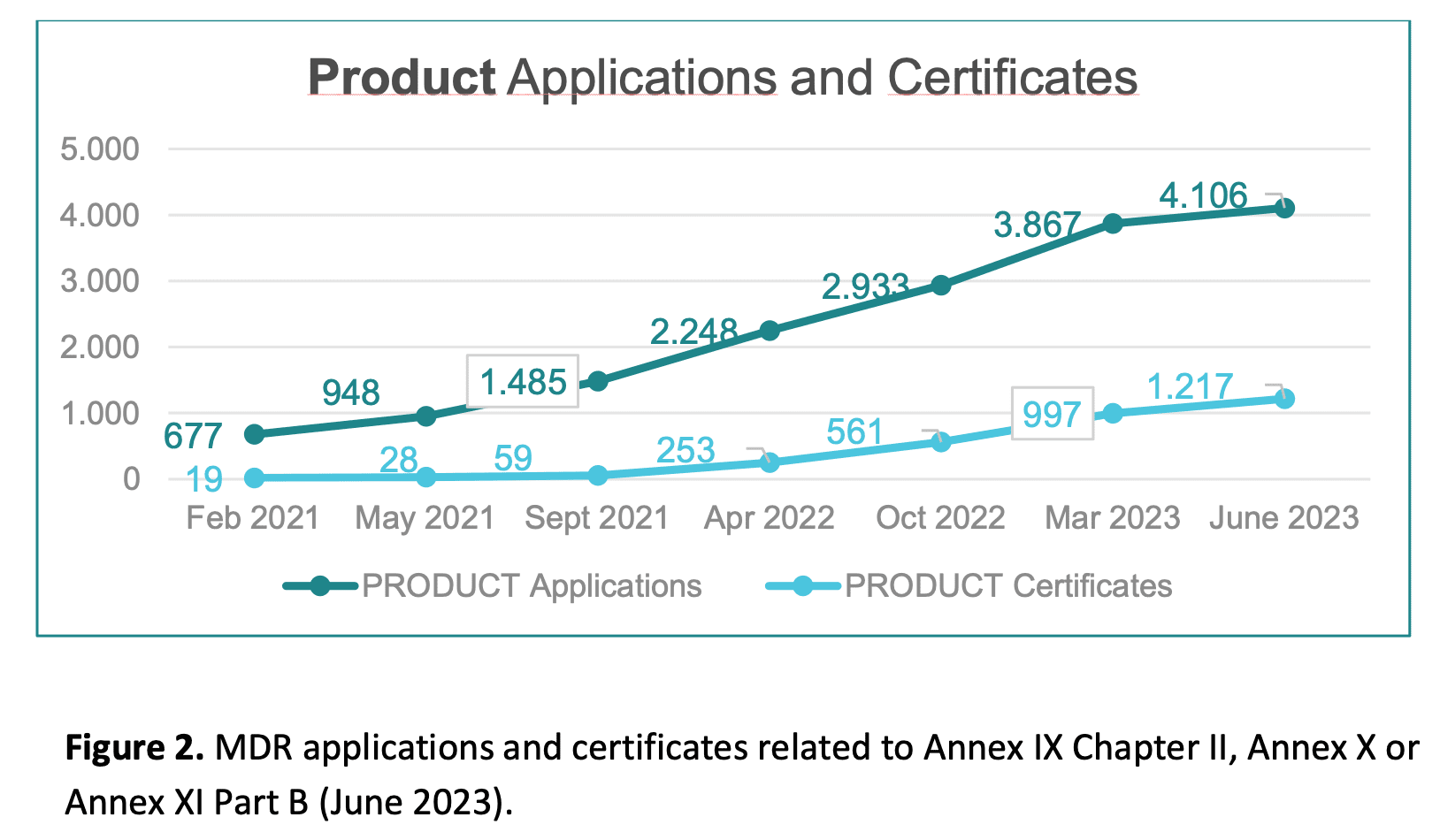
New issue of the MDCG Position Paper : MDCG 2022-11 revision 1 - Notice to manufacturers and NB
Medical devices regulation
📣 New issue of the MDCG Position Paper : MDCG 2022-11 revision 1
"Notice to manufacturers and notified bodies to ensure timely compliance with MDR and IVDR requirements"
For MD manufacturers
MDCG urges manufacturers to:
1️⃣ - Make the best possible use of the additional time provided by the amendments of the MDR and IVDR by submitting applications for conformity assessment in good time.
2️⃣ - Strengthen their efforts to transition as soon as possible and not to delay submissions further, as this could lead to bottlenecks in the work of notified bodies and possible shortages of products in the market.
3️⃣ - Regularly provide data on the situation regarding their devices.
For notified bodies
A new section “Call to notified bodies to streamline the certification process” has been created.
The MDCG calls on notified bodies to make the certification process more efficient, transparent and predictable. MDCG asks NB to:
1️⃣ - Improve their improve their conformity assessment activities in terms of transparency, timelines, predictability and consistency.
2️⃣ - Organise structured dialogues with manufacturers, which is expected to be part of the normal pre-application and conformity assessment activities and therefore not to be a separated service to be charged for. As a reminder, incomplete applications have been identified as an important cause of the delays.
3️⃣ - Regularly provide data on the situation regarding the certification of devices in order to monitor the progress of implementation of MDR and IVDR.
4️⃣ - Increase transparency around their capacity and timelines for conformity assessment and specifically to make publicly available…
We are at your service
Since the arrival of Regulation 2017/745, new requirements are requested and form part of the documentation to be presented to the notified body (e.g. review of the in-depth clinical evaluation, PMS procedure, QC results from validation products, proof of staff skills, etc.). CSDmed brings its expertise and a methodical approach to its clients, start-ups, manufacturers, importers and distributors of medical devices, thanks to a team of specialized experts and consultants, who will be able to address the MDR transition in its entirety.
🔗 Contact us and find out how we can help you.