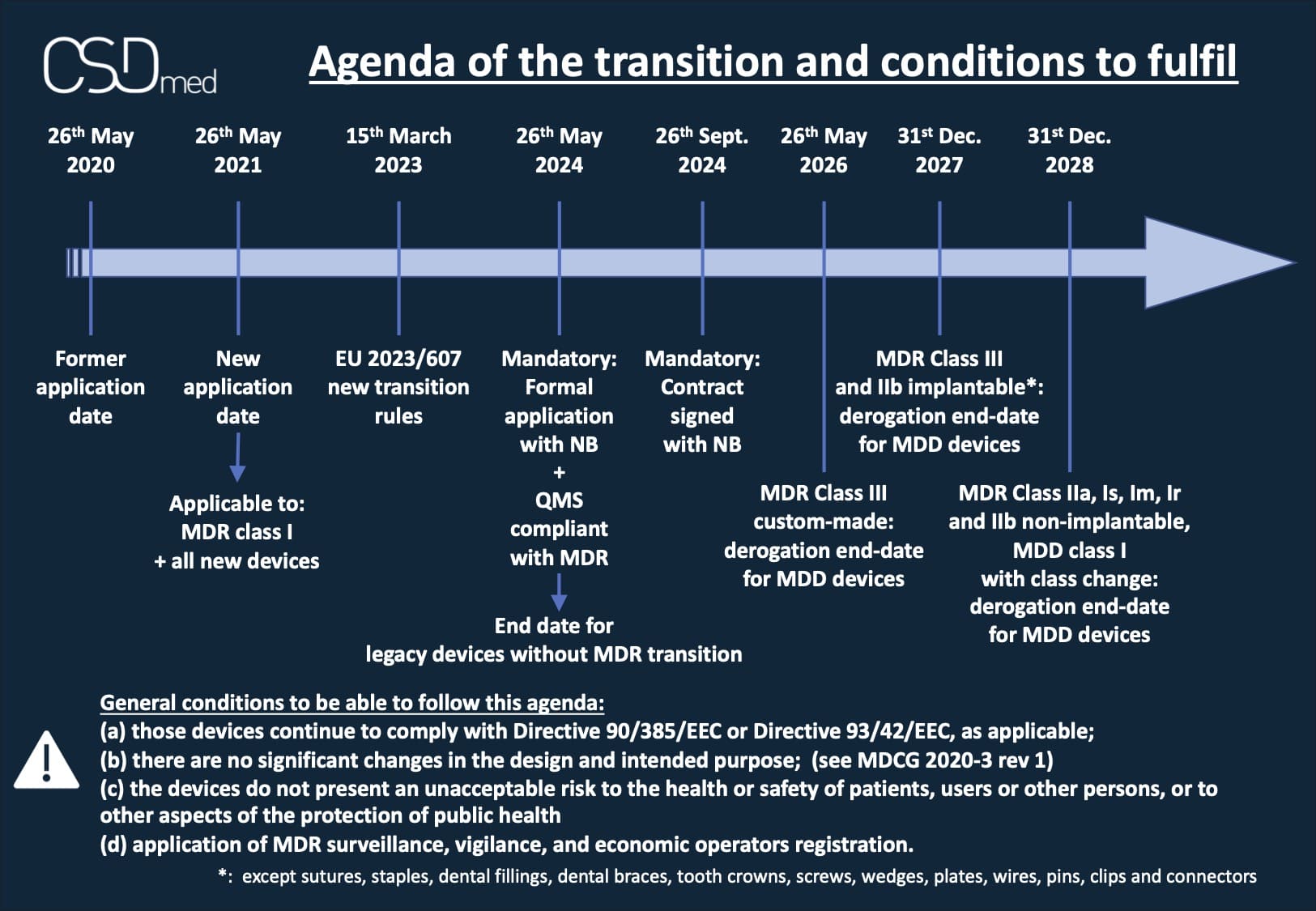
The calendar according to amending regulation 2023/607
Medical devices regulation
Many of our clients ask us questions about the timetable to take into account since the publication and entry into force (in accelerated procedure) of the amendment to MDR 2017/745, the famous EU 2023/607, applicable since March 15, 2023 .
Obligations to fulfil from the extended transition period:
Maintaining the validity of MDD certificates (or declaration of conformity):
- The certificate is valid as of May 26, 2021 and not withdrawn
- + if certificate expired on the date of entry into force of the amending regulation (03/15/2023):
- Contract signed with an ON in accordance with Annex VII, section 4.3, before expiration of MDD certificate of the MD concerned or a MD required to replace it* (Article 120, section 2a)
- A competent authority has granted an exemption according to Article 59 or requested the manufacturer to implement the applicable evaluation procedure according to Article 97 (Article 120, section 2b)
- For class I MDs under MDD that change class: Declaration of Conformity (DoC) valid as of May 26, 2021 (Article 120, section 3b)
*A device is intended to replace a device following significant change(s) in design, and/or performance, and/or intended use.
Conditions to be met for placing on the market:
- MDD Compliance (Article 120, section 3c - a)
- No significant change in purpose and/or design (Article 120, section 3c - b and see MDCG guide 2020-3 rev 1)
- No unacceptable risk (Article 120, section 3c - c)
- Implementation of an MDR-compliant QMS before May 26, 2024 (monitoring, vigilance, and registration of economic operators) - (Article 120, section 3c - d)
- Submission of a formal request to a Notified Body before May 26, 2024 and contract signed with the ON before September 26, 2024 (Article 120, section 3c - e)
- Application of Post-Market Surveillance (SAC), market surveillance, vigilance, registration of operators and products according to MDR (Article 120, section 3d)
If the conditions are met, the medical device intended to pass under MDR or intended to replace a MD under MDD will be placed on the market directly applicable.
Other contribution of amendment 2023/607
The amendment also repeals the end of the availability period.
How do I know that the manufacturer has the transition period?
- Current certificates under MDD will not be modified by Notified Bodies (“by law” extension)
- The manufacturer will be able to establish a “Self-declaration” - a model is available here
- The Notified Body may establish a “Confirmation letter” - a model is available here
- The competent authority may issue a “certificate of free sale”.
If the Notified Body selected for the MDR is different from the NB which had issued the certificate under MDD, a tripartite approval must be drawn up. Surveillance by the new ON under MDR will be done no later than September 26, 2024. The new ON will not be responsible for the previous surveillance) - Article 120 section 3e.
A model tripartite contract has been proposed by Team NB and is available here
What happens from 2029?
- All medical devices placed on the market must comply with Regulation MDR 2017/745
- Devices complying with the MDD present in distribution circuits or at the user's premises (sold or on deposit) can be sold/used without time limit other than expiration date.
- MDs that comply with the directive already installed at the user can continue to be used during their lifetime.
We are at your service
Since the arrival of Regulation 2017/745, new requirements have been requested and are part of the documentation to be presented to the notified body (e.g. review of the in-depth clinical evaluation, PMS procedure, QC results from validation products, proof of staff skills, etc.).
CSDmed brings its expertise and a methodical approach to its clients, start-ups, manufacturers, importers and distributors of medical devices, thanks to a team of specialized experts and consultants, who will be able to handle the MDR transition in its entirety.
🔗 Contact us and find out how we can help you.