The hidden risks of sourcing medical equipment from China
Medical devices regulation
🚫 The hidden risks of sourcing medical equipment from China 🚫
Importing medical equipment from China may seem attractive, but it hides major challenges and often underestimated risks:
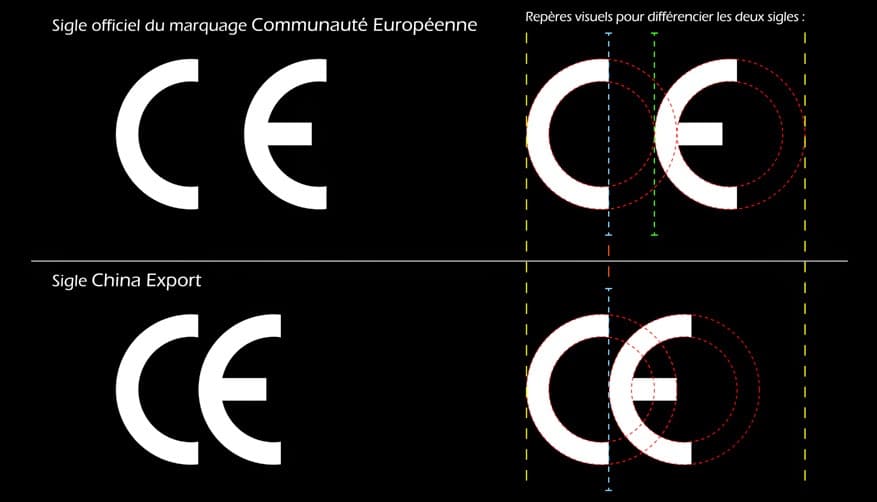
1. Non-compliance: Even with a CE mark, without the right validation process, it is easy to come across products that do not comply with European standards.
2. Liability and Recourse: Without a European representative, in the event of problems, who is responsible? Pursuing an overseas manufacturer can be costly and complex.
3. Sanctions and Regulations: The EU is strict. Importing or distributing without following protocol can result in severe fines and penalties.
4. Product Reliability: Without a local presence or sourcing expertise, how can you verify the quality or ensure the reliability of medical equipment?
What do European regulations say on this subject?
Chapter 2 of MDR 2017/745 describes the obligations of economic operators with regard to the importation of medical devices into Europe.
Here are some interesting articles regarding the importation of a medical device into Europe:
- Article 5: the medical device must comply with the regulation and meet the safety and performance requirements of Appendix I.
- Article 11: When the manufacturer of a device is not established in a Member State, the device may only be placed on the Union market if the manufacturer appoints a single authorized representative. The agent has obligations specified in Article 11.
- Article 13: Importers only place devices complying with this Regulation on the European Union market and must verify certain points before doing so (CE marking, EU declaration of conformity, labeling and UDI among others).
- Article 14: When making a device available on the market, distributors act, within the framework of their activities, with the diligence required to comply with the applicable requirements. They also have obligations just like the authorized representative or the importer.
- Article 16: A distributor, importer or other natural or legal person fulfills the obligations incumbent on manufacturers if they carry out one of the following tasks:
- a) make a device available on the market under its own name, under its company name or under its registered trademark, unless a distributor or importer concludes an agreement with the manufacturer according to which the latter is mentioned as such on the label and remains responsible for compliance with the requirements imposed on manufacturers by this regulation;
- b) change the destination of a device already placed on the market or put into service;
- (c) modify a device already placed on the market or put into service in such a way that this may affect compliance with the applicable requirements.
✨ The SOLUTION: choose a trusted partner ✨
With CSDmed, we:
- Avoid the Pitfalls: We guide you through the sourcing maze, avoiding costly mistakes.
- Ensuring Compliance: We meticulously check each product to ensure that it complies with all European standards and regulations.
Do not play with conformity and quality. Choose a trusted partner for transparent and reliable medical equipment sourcing in China.
🔗 Contact us and find out how we can protect you and boost your business.